7+ bromine bohr diagram
The electron configuration of an element with an atomic number greater than 18 cannot be properly determined according to the Bohr atomic model. It is relatively unreactive.

Bromine By Kaylie Hutzell
Carbon is above it.

. It is the rarest naturally occurring element in the Earths crust occurring only as the decay product of various heavier elements. Properties of ceramics Classification of ceramics Ceramic raw material Fabricating and processing of ceramic Application of Ceramics 1 Glasses 2 Clay Products 21 Structural clay product 22. The Bohr Model of NitrogenN has a nucleus that contains 7 neutrons and 7 protons.
Harraz Presentation Ceramic Materials Hassan Z. Bromine Br is in group 17 so it has 7 valence. The Bohr Model of MagnesiumMg has a nucleus that contains 12 neutrons and 12 protons.
It is a hard brittle crystalline solid with a blue-grey metallic luster and is a tetravalent metalloid and semiconductorIt is a member of group 14 in the periodic table. Bohr model or RutherfordBohr diagram presented by Niels Bohr and Ernest Rutherford in 1913. Astatine is a chemical element with the symbol At and atomic number 85.
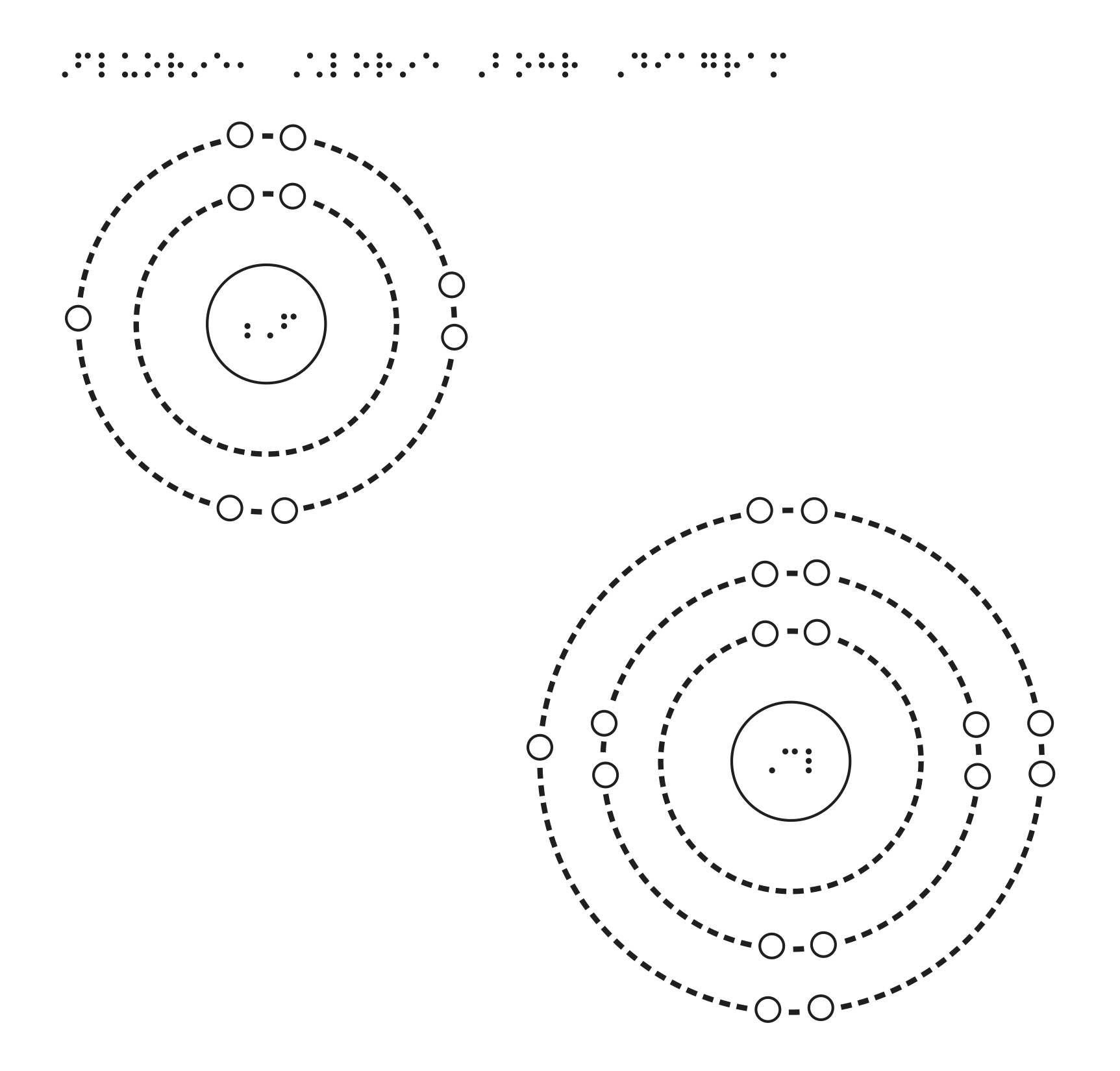
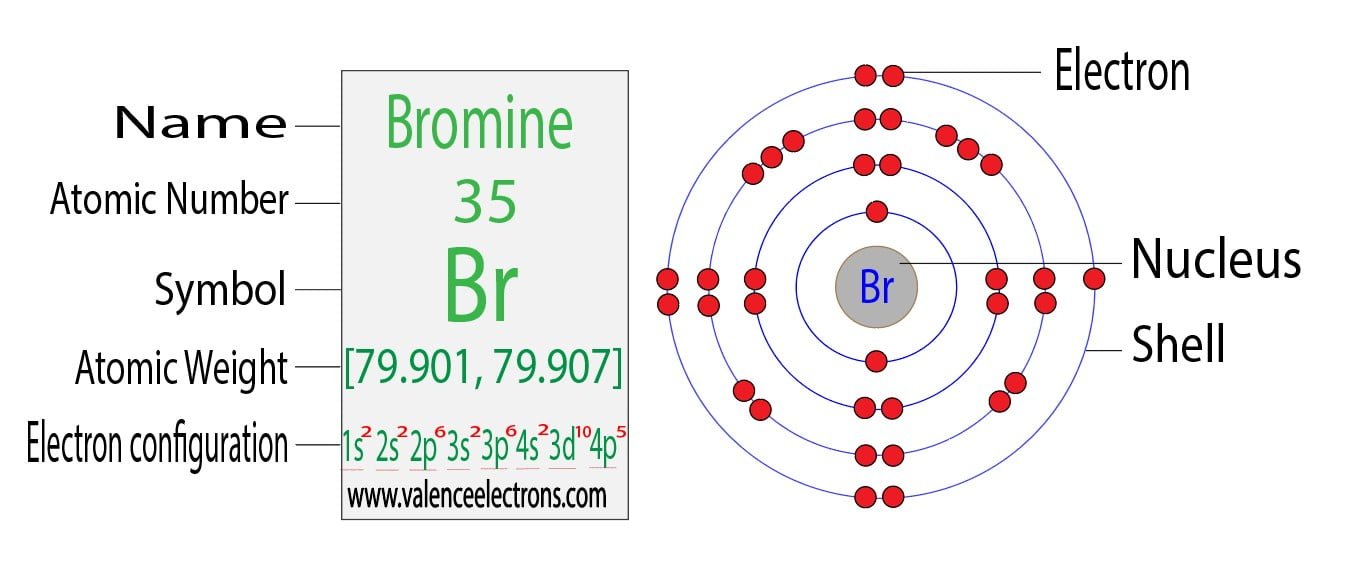
This nucleus is surrounded by two-electron shells named K-shell and L-shell. The formation of halogen-bonded complexes involving bromine and chlorine as electron acceptor species was first. Therefore the order of the number of electrons in each shell of the bromineBr atom is 2 8 18 7.
Because of its high. Electrons arrangement in Bromine or Bohr model of Bromine. You will get the detailed information about the periodic table which will convert a newbie into pro.
You will also get the HD images of the Periodic table for FREE. For example sodium Na is in group 1 therefore it has 1 valence electron. The most stable is astatine-210 with a half-life of 81 hours.
Give the total number of electrons and the number of valence electrons for each element listed below. Dp level on the 0001 electronbohr 3 molecular surfaces of the XB donors and acceptors. To write the orbital diagram of sodiumNa you have to do the electron configuration of sodium.
The photon energy of the emitted photon is equal to the energy difference between the two states. 296 Pauling scale Crystal structure of. The periodic table of the chemical elements is a tabular method of displaying the chemical elements.
The temperature dependence of all critical points provided a benchmark for the interband electronic transition. It is a member of the chalcogen group in the periodic table a highly reactive nonmetal and an oxidizing agent that readily forms oxides with most elements as well as with other compoundsOxygen is Earths most abundant element and after hydrogen and helium it is the third-most abundant element in the. This nucleus is surrounded by three-electron shells named K-shell L-shell and M-shell.
We investigated the complex dielectric function of bromide perovskite single crystals MAPbBr3 FAPbBr3 and CsPbBr3 by spectroscopic ellipsometry from 1 to 5 eV in the 183423 K temperature range and under a dry nitrogen environment. A sample of the pure element has never been assembled because any. Although precursors to this table exist its invention is generally credited to Russian chemist Dmitri Mendeleev in 1869.
1s is the closest and lowest energy orbital to the nucleus. This nucleus is surrounded by three-electron shells named K-shell L-shell and M-shell. Iron ˈ aɪ ər n is a chemical element with symbol Fe from Latin.
Silicon is a chemical element with the symbol Si and atomic number 14. The outermost shell in the Bohr diagram of Nitrogen contains 5 electrons that also called valence electrons. The Bohr Model of OxygenO has a nucleus that contains 8 neutrons and 8 protons.
And Paulis exclusion principle is that the value of four quantum numbers of two electrons in an atom cannot be the same. Bohrs diagram of Helium has only one electron shell K-shell. There are many possible electron transitions for.
FCl Cl 2 ClBr Br 2 and ClI with several Lewis bases Y. The outermost shell in the Bohr diagram of Carbon contains 4 electrons that also called valence electrons. 2 8 18 7.
OUTLINE OF TOPIC 3. The electron configuration of an element with an atomic number greater than 18 cannot be properly determined according to the Bohr atomic model. The outermost shell in the Bohr diagram of Aluminum contains 3 electrons that also called valence electrons.
The Bohr Model of CarbonC has a nucleus that contains 6 neutrons and 6 protons. It is a metal that belongs to the first transition series and group 8 of the periodic tableIt is by mass the most common element on Earth right in front of oxygen 321 and 301 respectively forming much of Earths outer and inner coreIt is the fourth most common. Electrons can be arranged correctly through orbits from elements 1 to 18.
Ferrum and atomic number 26. From the Bohr diagram of an atom we can easily find the number of valence electrons in an atom by looking at its outermost shell. Electronic configuration of Bromine Ar 3d 10 4s 2 4p 5.
In this diagram the N Y scale was defined by setting E ClI 10 and. 54 Acid reactions in everyday life 55 Alkalis and bases 56 Characteristic reactions of acids 57 Acids and alkalis in chemical analysis 58 Salts 59 Preparing soluble salts 510 Preparing. Oxygen O is in group 16 therefore it has 6 valence electrons.
So we have to find a valence electron in the Helium atom for this look at its Bohr diagram. Oxygen is the chemical element with the symbol O and atomic number 8. The electron configuration of all the elements can be done through the orbital diagram.
The outermost shell in the Bohr diagram of Oxygen contains 6 electrons that also called valence electrons. This nucleus is surrounded by two-electron shells named K-shell and L-shell. Electrons can be arranged correctly through orbits from elements 1 to 18.
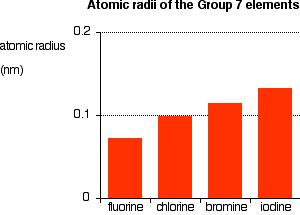
The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to an electron making a transition from a high energy state to a lower energy state. Atomic radius of Bromine. The Bohr Model of ChlorineCl has a nucleus that contains 18 neutrons and 17 protons.
183 picometers van der Waals radius Valence electrons in Bromine. This nucleus is surrounded by three-electron shells named K-shell L-shell and M-shell. The outermost shell in the Bohr diagram of Chlorine contains 7 electrons that also called valence electrons.
This nucleus is surrounded by two-electron shells named K-shell and L-shell. Which has been discussed in detail above. The Bohr Model of AluminumAl has a nucleus that contains 14 neutrons and 13 protons.
The outermost shell in the Bohr diagram of Magnesium contains 2 electrons that also called valence electrons. And germanium tin lead and flerovium are below it. Bohr Diagram Physical Science NamePerDue date_____ Valence Electron Practice.
All of astatines isotopes are short-lived. Bohr model of an atom consists of a small nucleus that contains protons and neutrons this nucleus is surrounded by different electron shells or energy levels where electrons are revolved in a definite circular path similar to the structure of the Solar System. 1st Ionization energy of Bromine.

Single Molecule Magnets Novel Mn8 And Mn9 Carboxylate Clusters Containing An Unusual Pentadentate Ligand Derived From Pyridine 2 6 Dimethanol Inorganic Chemistry

Bromine By Kaylie Hutzell
Atomic And Physical Properties Of Periodic Table Group 7 The Halogens
Ch104 Chapter 2 Atoms And The Periodic Table Chemistry

Nitrogen Bohr Model How To Draw Bohr Diagram For Nitrogen N Atom
Bohr Diagrams Resource Imageshare

General Chemistry Print Version Wikibooks Open Books For An Open World

Bohr Rutherford Diagram For Bromine Br Youtube

Bohr Rutherford Diagrams Lewis Dot Diagrams Eve Wongworakul Chemistry Unit

Bromine Br On Twitter This Is The Bohr Model For Bromine Element That Shows The 35 Protons Electrons And 45 Neutrons It Also Shows The Number Of Valence Electrons Which Is 7 The
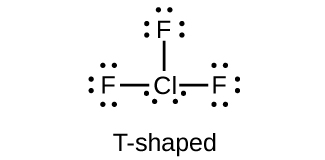
Occurrence Preparation And Properties Of Halogens Chemistry

Bromine Br

Bromine Bohr Model How To Draw Bohr Diagram For Bromine Br Atom

Extended Periodic Table Wikipedia

Bromine Bohr Model How To Draw Bohr Diagram For Bromine Br Atom

Beryllium Bohr Model How To Draw Bohr Diagram For Beryllium Be Atom
Bromine Br Electron Configuration And Orbital Diagram